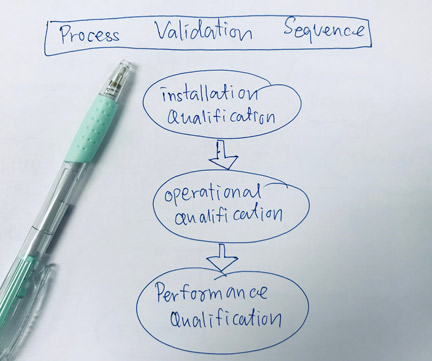
GST Enterprises executes commissioning of start-up production facilities, process equipment and control systems . Our approach to qualification incorporates our in-depth understanding of global regulatory guidelines (USFDA, EMA, MHRA, Health Canada, WHO). We provide onsite / offsite C&Q services for BioPharma, Vaccines, Oral Solid Dosage, Fill / Finish facilities.
To Know more please write to us at info@gst-enterprises.com
GST Enterprises is an engineering company specialized in producing and designing innovative technological solutions and high-quality products for the pharmaceutical, chemical and biotechnological industries.